The National Agency for Food and Drugs Administration and Control (NAFDAC) is notifying the public that the Philippines Food and Drug Administration (FDA) has warned against the purchase and use of the five unregistered drug products namely:
- Niaosu Ruangao,
- Compound Bismuth Subnitrate Tablets,
- Cimetidine Injection 2ml:0.2g Ampoule,
- OTC Dextromethorphan Hydrobromide Syrups,
- Jingxin Pharmceutical Roxithromycin Capsules 0.15g
- Lean Slim Appetite Suppressant Strong Capsule 30’s
- Lean Slim Appetite Suppressant Mild Capsule 30’s
- Dianne ® – 35 Cyproterone acetate 2.00 mg/ Ethinylestradiol 0.035 mg Sugar-Coated Tablet
- Androcur® Siproteron asetat 50 mg Tablet
- Androcur TM (Cyproterone acetate) 50 mg Tablet
- Testoviron Depot (Testosterone enanthate) 250 mg, 1mL Ampoule
- Climen® Estradiol valerat/Siproteron asetat 2mg+2mg/1mg Kapli Tablet
- Elleacnelle® Siproteron asetat 2000 mcg/ Etinilestradiol 35 mcg Kapli Tablet
According to the Philippines FDA, the above-mentioned drug products have not gone through the registration process of the FDA and have not been issued with proper authorization in the form of Certificate of Product Registration. Thus, the FDA cannot guarantee their quality, safety and efficacy. Therefore, consumption of such violative products may pose potential danger or injury to health.
Risk Statement
The illegal marketing of unregistered medicines or falsified drug products poses a risk to the health of people, since by not complying with the process for marketing authorization, legal importation, distribution or sale, the safety, quality and efficacy of the products are not guaranteed.
The administration of the falsified or unregistered injection products may cause harm to patients or lead to fatal consequences such as death.
These products are obviously not registered by NAFDAC and not available in the NAFDAC database. It is important to ensure that they are not smuggled into the country through informal markets.
NAFDAC implores importers, distributors, retailers and healthcare providers to always exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale or use of the falsified or unregistered medicinal products. All medicinal products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of adverse drug reaction, substandard and falsified medicines to the nearest NAFDAC office, NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of the medicinal product to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med-safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
NAFDAC……….Customer-focused, Agency-minded!!!
Signed Management
SEE PRODUCTS PHOTOS ATTACHED BELOW
Product Photos
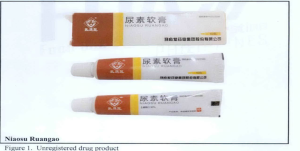
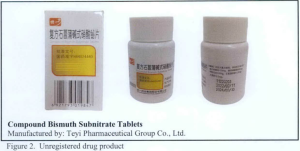
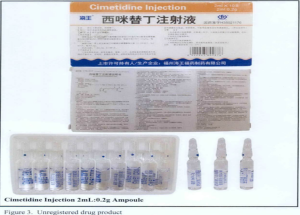
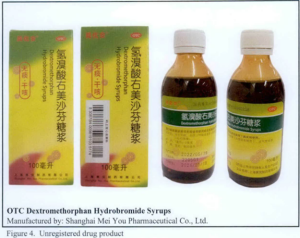
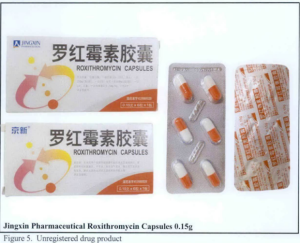
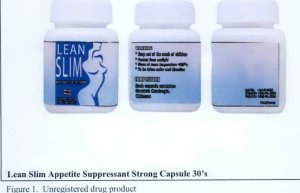
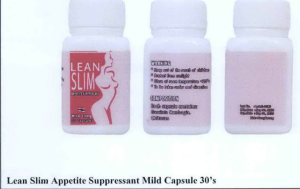
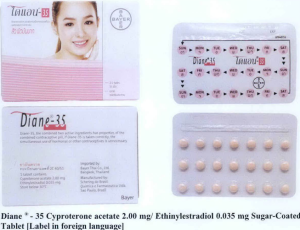
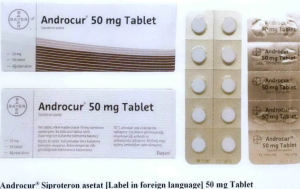
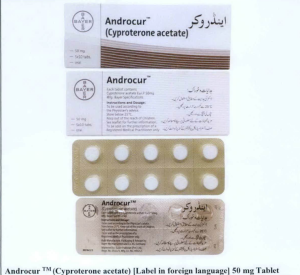
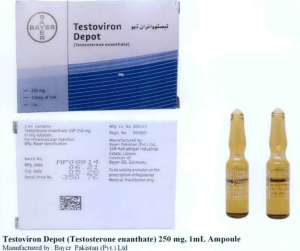
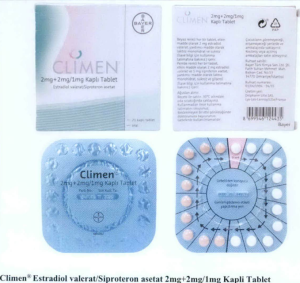
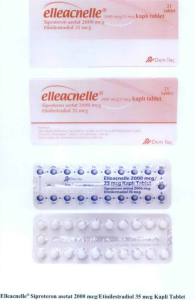
The following are photos of the implicated products as uploaded by the Philippines FDA;
The National Agency for Food and Drugs Administration and Control (NAFDAC) is notifying the public that the Philippines Food and Drug Administration (FDA) has warned against the purchase and use of the five unregistered drug products namely:
- Niaosu Ruangao,
- Compound Bismuth Subnitrate Tablets,
- Cimetidine Injection 2ml:0.2g Ampoule,
- OTC Dextromethorphan Hydrobromide Syrups,
- Jingxin Pharmceutical Roxithromycin Capsules 0.15g
- Lean Slim Appetite Suppressant Strong Capsule 30’s
- Lean Slim Appetite Suppressant Mild Capsule 30’s
- Dianne ® – 35 Cyproterone acetate 2.00 mg/ Ethinylestradiol 0.035 mg Sugar-Coated Tablet
- Androcur® Siproteron asetat 50 mg Tablet
- Androcur TM (Cyproterone acetate) 50 mg Tablet
- Testoviron Depot (Testosterone enanthate) 250 mg, 1mL Ampoule
- Climen® Estradiol valerat/Siproteron asetat 2mg+2mg/1mg Kapli Tablet
- Elleacnelle® Siproteron asetat 2000 mcg/ Etinilestradiol 35 mcg Kapli Tablet
According to the Philippines FDA, the above-mentioned drug products have not gone through the registration process of the FDA and have not been issued with proper authorization in the form of Certificate of Product Registration. Thus, the FDA cannot guarantee their quality, safety and efficacy. Therefore, consumption of such violative products may pose potential danger or injury to health.
Risk Statement
The illegal marketing of unregistered medicines or falsified drug products poses a risk to the health of people, since by not complying with the process for marketing authorization, legal importation, distribution or sale, the safety, quality and efficacy of the products are not guaranteed.
The administration of the falsified or unregistered injection products may cause harm to patients or lead to fatal consequences such as death.
These products are obviously not registered by NAFDAC and not available in the NAFDAC database. It is important to ensure that they are not smuggled into the country through informal markets.
NAFDAC implores importers, distributors, retailers and healthcare providers to always exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale or use of the falsified or unregistered medicinal products. All medicinal products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of adverse drug reaction, substandard and falsified medicines to the nearest NAFDAC office, NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of the medicinal product to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med-safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
NAFDAC……….Customer-focused, Agency-minded!!!
Signed Management
SEE PRODUCTS PHOTOS ATTACHED BELOW
Product Photos
The following are photos of the implicated products as uploaded by the Philippines FDA;






