

- Events, Home Featured


- Drug Spotlight, Drugs Programmes, Home Featured


- Home Featured, Recalls and Safety Alerts


- Drug Spotlight, Drugs Programmes, Home Featured
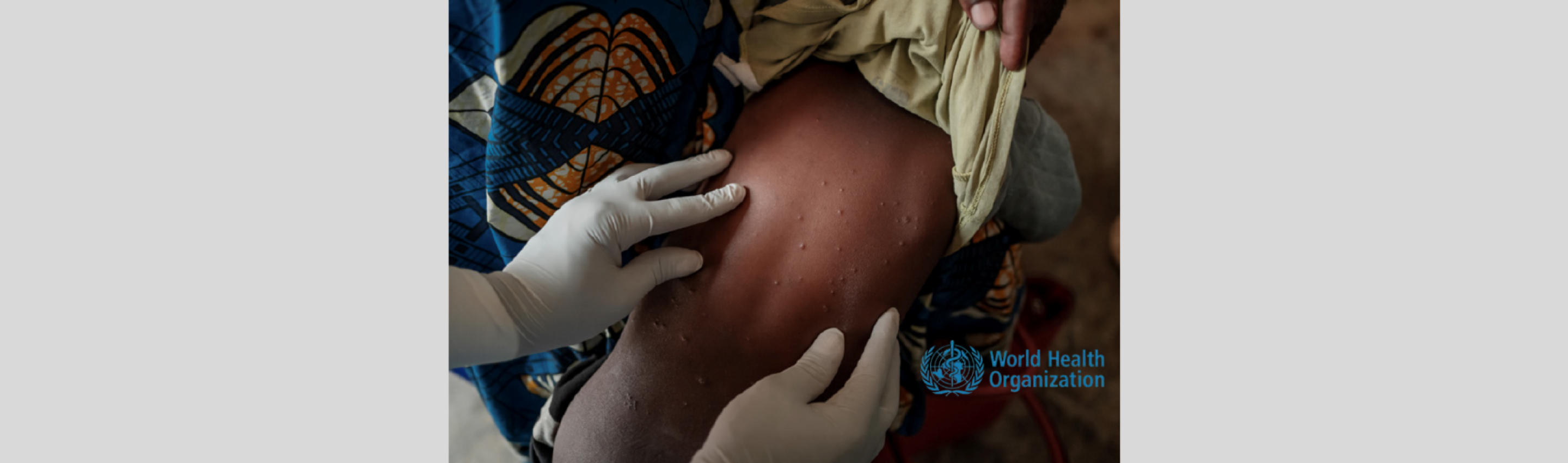
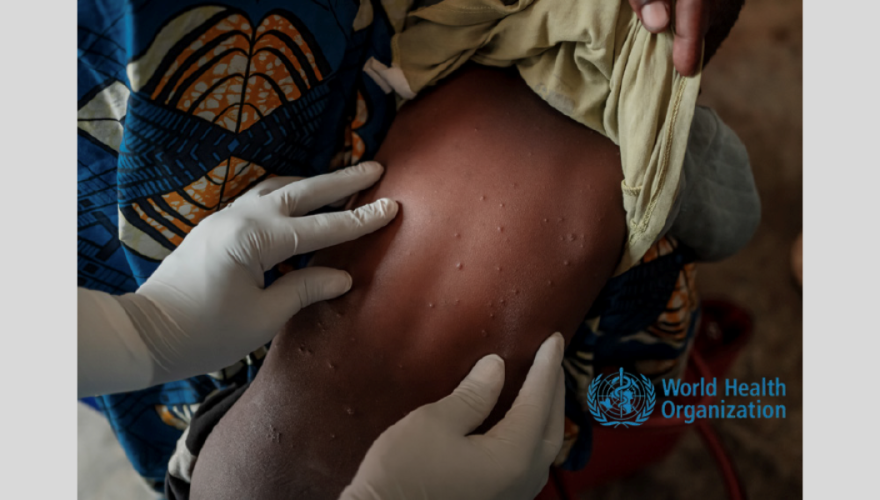
- Home Featured, Press Release


- Home Featured
Launch of the Implementation of Strategy and Roadmap for Trans-fatty…
Opening Remarks by Prof. Mojisola Christianah Adeyeye, at the World…
World AMR Awareness Week (WAAW) 2025
World Food Safety Day (WFSD) 2025
Peer Reviewed Publications:
- Aderibigbe, O. O., Adamu, B. B., Osaji, I. O., Babarinde, A. O., Nwachukwu, C. U., Adegboye, A., … & Adeyeye, M. C. (2023). Secondary Validation of a Liquid Scintillation Counter-method for Residues of Tetracyclines, Beta-lactams, and Sulfonamides in Seafood/Aquaculture Products. Journal of Food Protection, 86(4), 100055.
- Adeyeye, M., Olasupo S., Akinyemi, A., Kayode, J., & Sonny-Afoekelu, U (2024). Impact of Digitalization in strengthening the regulatory functions of NAFDAC: Opportunities and Challenges for other National Regulatory Authorities. Journal of Regulatory Science, 12(1). Partly funded by Internally Generated Revenue, Global Fund, BMGF, WHO& USAID
- Kanu, N. M., Adeyeye, C. M., Abiola, V. O., Adekunle–Segun, O., Adegoke, E. M., Akapo, A., & Okezue, M. A. (2024). The safety, quality evaluation, and lot release of COVID-19 vaccines imported and used in Nigeria from march 2021 to march 2022. Discover Health Systems, 3(1), 15. Funded by Internally Generated Revenue
- Adeyeye, M., Kayode, J., Adeniran, A., Osho, F., & Udokwelu, W. (2023). Enabling Pharmaceutical Traceability in The Nigerian Supply Chain using GS1 Global Standards: Lean Traceability Including In-Country Serialization of COVID-19 Vaccines. Journal of Regulatory Science, 11(1), 1-14. Partly funded by Internally Generated Revenue, Global Fund, BMGF, WHO& USAID
- Elemuwa, U. G., Bitrus, F., Oreagba, I. A., Osakwe, A. I., Abiodun, A. S., Onu, K., … & Adeyeye, C. M. (2024). Trends in Adverse Event Reporting Before and After the Introduction of the Med Safety App in Nigeria. Pharmaceutical Medicine, 1-9.
Recalls and Safety Alerts
These are recalls and safety alerts released by NAFDAC. The alert notices and safety communications contain safety measures to be taken and information that may impact both treatment and diagnostic choices for healthcare providers and patients.
Blacklisted Products
These products are approved for withdrawal, suspension and cancellation by NAFDAC. They are therefore no longer permitted for manufacture, importation, exportation, distribution, advertisement, sale and use within Nigeria.
NAFDAC Updates
Our Services
Meet the Director General.
Prof Moji Adeyeye is the Director General of National Agency for Food and Drug Administration and Control (NAFDAC) where she has added strong governance structure, regulatory system strengthening, and strengthening of the local pharmaceutical companies through international best practices with emphasis on local manufacturing.

Prof. Moji Christianah Adeyeye
Director General, NAFDAC



