Emergency use authorization of the Mpox vaccine by NAFDAC: A crucial step in Nigeria’s public health response
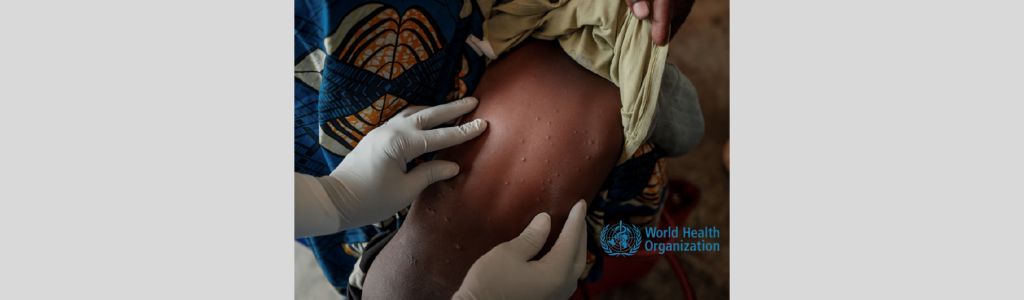
The 2022 Monkeypox (Mpox) outbreak, initially detected in the United Kingdom, posed a significant global health challenge. The World Health Organization’s declaration of a Public Health Emergency of International Concern underscored the urgency of the situation. In Nigeria, the National Agency for Food and Drug Administration and Control (NAFDAC) took proactive measures to ensure the […]
Public Alert No. 037/2024 – Alert on Confirmed Counterfeit of Mabthera 500mg/50ml in Nigeria
The National Agency for Food and Drugs Administration and Control (NAFDAC) informs the healthcare providers and the public of a report of Confirmed Counterfeit of Mabthera 500mg/50ml with batch number N7458B07 in Nigeria. The Marketing Authorization Holder (MAH) Roche received a call from a patient to enquire the genuineness of the product before purchasing it. […]
Public Alert No. 036/2024 – Sale and Distribution of Unregistered/Banned Veterinary Products
The National Agency for Food and Drug Administration and Control (NAFDAC) wishes to inform the public about the distribution and sales of unregistered veterinary drugs and veterinary drugs banned for use in food-producing animals. Following a report received from the Veterinary Medicines and Allied Products (VMAP) Directorate, some establishments listed below have been identified to […]
Public Alert No. 035/2024 – Alert on the Recall of Dove Beauty Cream Bar Soap due to Butyphenyl Methylpropional (BMHCA) content
The National Agency for Food and Drug Administration and Control (NAFDAC) is alerting the public about the recall of Dove Beauty Cream Bar Soap (100g) with batch number 81832M 08, produced in Germany, due to chemical impurity. The product does not comply with the Cosmetic Products Regulation as it is said to contain Butylphenyl Methylpropional […]
Public Alert No. 034/2024 – Philippines Authority Warns Against the Purchase and Use of Some Unregistered Drug Products in Philippines
The National Agency for Food and Drugs Administration and Control (NAFDAC) is notifying the public that the Philippines Food and Drug Administration (FDA) has warned against the purchase and use of the five unregistered drug products namely: Niaosu Ruangao, Compound Bismuth Subnitrate Tablets, Cimetidine Injection 2ml:0.2g Ampoule, OTC Dextromethorphan Hydrobromide Syrups, Jingxin Pharmceutical Roxithromycin Capsules […]
Public Alert No. 033/2024 – Alert on the Recall of Radox Care + Moisturise Liquid Hand Wash due to presence of Butyphenyl Methylpropional (BMHCA) Content
The National Agency for Food and Drugs Administration and Control (NAFDAC) is notifying the public of the recall of Radox Care + Moisturise Liquid Hand Wash by the European Union (EU) in Brussels, Belgium. The product does not comply with the Cosmetic Products Regulation as it is said to contain Butyphenyl Methylpropional (BMHCA) which is […]
Public Alert No. 032/2024 – Singapore to Ban Formaldehyde in Interior Paints over health concerns by 2026
The National Agency for Food and Drugs Administration and Control (NAFDAC) is notifying the public that Singapore’s National Environment Agency (NEA) is banning the sale of indoor paints containing formaldehyde from 1 January 2026. To ensure compliance, paint manufacturers and importers will be mandated to submit test reports demonstrating that the formaldehyde content in their […]
Press Briefing on WHO PQ of Swiss Pharma{Swipha}‘s Second Product Antimalarial: Sulfadoxine/Pyrimethanine Tablets {SP Combination}
PROTOCOL The National Agency for Food and Drug Administration and Control (NAFDAC) was set up by act of the parliament (Act Cap N1 LFN 2004 as amended) to regulate and control the manufacture, importation, exportation, distribution, advertisement, sale and use of Food, Drugs, Cosmetics, Medical Devices, Packaged Water, Chemicals and Detergents” (collectively known as regulated […]
Remarks and Talking Points of Prof. Mojisola Christianah Adeyeye Phd, Fas; at the NAFDAC Export Stakeholder Interactive Session on NAFDAC Export Regulations
Remarks by Prof. Adeyeye C M, Director General NAFDAC NAFDAC Export Stakeholder Interactive Session on NAFDAC Export Regulations NAFDAC PID Conference Centre, Yaba, Lagos 20th May, 2024. PROTOCOLI was quite curious to find out today what exactly the Cocoa Stakeholders are concerned with, especially in the draft Cocoa regulations we have posted for comments. First, let […]
Public Alert No. 031/2024 – Illegal Sale of Substandard and Falsified (SF) Colamar (Artemether/Lumefantrine 20/120mg) Oral Suspension
The National Agency for Food and Drugs Administration and Control (NAFDAC) informs the public of the illegal distribution and marketing of a substandard and falsified anti-malaria, Colamar (Artemether/Lumefantrine 20/120mg). The Substandard and falsified product is a pass-off of Lonart Suspension and has a fake NAFDAC Registration Number (NRN): B4-4065 which is a registration number of Malasyn Tablets […]
