The National Agency for Food and Drugs Administration and Control (NAFDAC) is informing the public of an investigational report from BBC revealing that an Indian pharmaceutical company Aveo Pharmaceuticals is manufacturing unlicensed, highly addictive opioids and exporting them illegally to some West African countries including Ghana, Nigeria, and Cote D’Ivoire, where they are constituting a major public health crisis.
Aveo Pharmaceuticals is based in Mumbai and makes a range of pills that go under different brand names and are packaged to look like legitimate medicines. These falsified products contain harmful combinations ingredients, namely tapentadol and carisoprodol.
Tapentadol is a powerful opioid, and carisoprodol is a muscle relaxant, so addictive, that it is banned in Europe.
The combination of the two drugs is not licensed for use anywhere in the world as they can cause breathing difficulties and seizures and an overdose can kill. Despite the risks, these opioids are popular as street drugs in many West African countries, because they are so cheap and widely available.
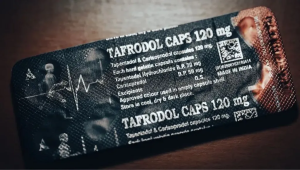
According to the BBC World Service packets of green pills labelled Tafrodol, branded with the Aveo logo, were found for sale on the streets of Ghanaian, Nigerian, and Ivoirian towns and cities.
Product details
The details of the product are as follows;
Product Brand Name: Tafrodol
Product Manufacturer: Aveo Pharmaceuticals, Mumbai, India.
Product Photo

Surveillance activities have been heightened in the Ports and States to stop the importation, distribution, and sale of these pills on the streets.
All NAFDAC zonal directors and state coordinators have been directed to carry out surveillance activities and mop up all within the zones and states.
Importers, distributors, retailers, and consumers are hereby advised to immediately stop the importation, distribution, sale and use of all these illegally imported opiods.
Healthcare professionals and consumers are advised to report any suspicion of sale of these products, substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
NAFDAC………. Customer-focused, Agency-minded!!!






