The National Agency for Food and Drugs Administration and Control (NAFDAC) is notifying the public about the circulation of a confirmed falsified Cikatem (Artemether 180mg/Lumefantrine 1080mg) suspension. This product was discovered in the Coordinated Wholesale Centre (CWC) in Kano following a consumer complaint received and investigated by the post marketing surveillance (PMS) officers of the Agency in Kano. All the falsified Cikatem (Artemether 180mg/Lumefantrine 1080mg) suspension discovered during the investigation has been mopped up and surveillance by the PMS Directorate is still ongoing.
The product was observed to have a discrepancy on the product packaging with NAFDAC Registration Number (A11-100025) printed on the label, which belonged to Cikatem Tablet 20/120mg, and not the suspension formulation.
The manufacturing facility of the product was visited for further investigation, during which 17,280 bottles of Cikatem (Artemether 180mg/Lumefantrine 1080mg) suspension were placed on hold and are scheduled for destruction.
ARTEMETHER+LUMEFANTRINE FOR ORAL SUSPENSION is indicated for the treatment of malaria in children caused by all forms of plasmodium including severe malaria.
Risk Statement
Counterfeit or falsified medicines endanger people’s health because they do not comply with regulatory standards, which means the safety, quality, and efficacy of these products are not ensured. The use of counterfeit medicines often fails to effectively treat diseases or conditions, leading to serious health consequences, including death.
Product details
The product details are listed below:
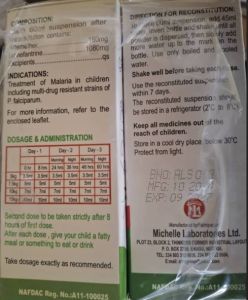
Product Name: Cikatem Suspension (Artemether 180mg/Lumefantrine 1080mg)
Batch No: ALS063
Mfg. Date: 10/2024
Exp. Date: 09/2027
NRN NO: NRN A11-100025
Manufactured by: Michelle Laboratories
Manufacturer Address: Plot 23, Block 2, Thinkers Corner Industrial Layout P.O. Box 2709, Enugu, Nigeria.
Product picture


All NAFDAC zonal directors and state coordinators have been directed to carry out surveillance and mop up the counterfeit products within the zones and states.
Distributors, retailers, healthcare professionals, and caregivers are hereby advised to exercise caution and vigilance within the supply chain to avoid the distribution, sale, and use of counterfeit products. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
NAFDAC………. Customer-focused, Agency-minded!!!
