The National Agency for Food and Drug Administration and Control (NAFDAC) is alerting the public about a recall of some batches of multivitamins and iron Supplements in the USA.
These brands of vitamins, known as CINCINNATI (WKRC), sold nationwide at retailers such as Walmart, Amazon, and Target, are being recalled due to “risk of serious injury or death” from poisoning, especially for children.
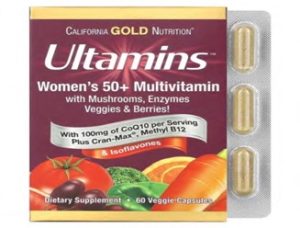
According to a recall report from the United States Consumer Product Safety Commission, around 60,000 units of iHerb/California Gold Nutrition Iron Supplements Multivitamins that were recalled are: Daily Prenatal Multi, Ultamins Women’s Multivitamin, and Ultamins Women’s 50+ Multivitamin.
The authorities discovered that the packages lack child-resistant packaging, which poses a risk of fatal poisoning if young children swallow the contents.
Risk Statement
Due to a lack of compliance with child-resistant standards, administering these iron-containing dietary supplements poses a risk of deadly poisoning if young children access and ingest the contents. Iron supplements, even a few tablets, can be highly toxic to young children, potentially causing severe symptoms such as vomiting, stomach pain, low blood pressure, bleeding, or liver damage. In serious cases, symptoms escalate quickly and can become life-threatening.
Product details
The details of the affected batches are as follows:
Product Name
| Daily Prenatal Multi
| Ultamins Women’s Multivitamin
| Ultamins Women’s 50+ Multivitamin |
Product Manufacturer: | iHerb/ California Gold Nutrition | iHerb/ California Gold Nutrition | iHerb/ California Gold Nutrition |
Batch Codes: | 2307050A, 2404096A, 2411100A | V0532, V0533 | V0534, V0536 |
Expiration Date: | 08/2025, 11/2026 | 11/2026, 07/2026 | 07/2026, 11/2026 |
PRODUCT Images


All NAFDAC zonal directors and state coordinators have been directed to carry out surveillance and mop up the CINCINNATI (WKRC) product within the zones and states.
Distributors, retailers, healthcare professionals, and caregivers are advised to exercise caution and vigilance in the supply chain to prevent the distribution, sale, and use of the products. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322, or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
NAFDAC………. Customer-focused, Agency-minded!!!
