The National Agency for Food and Drug Administration and Control (NAFDAC) hereby alerts the public about an unregistered Gold Vision Oxytocin injection 10IU with a fake NAFDAC Reg. No. A4-9566. The product is purportedly manufactured by Anhui Hongye Pharmaceutical Co., Ltd., Fengyang East Road, Bengbu, Anhui Province, China, and marketed by Gold Vision Medicals, No.4 Range Avenue, Independence Layout, Enugu, Nigeria.
The product was discovered during a Risk-Based sampling survey activity conducted by officers of the Post Marketing Surveillance (PMS) directorate of NAFDAC. Further investigation revealed that three (3) falsified products, namely A-tocin injection, Extocin Injection, and Claxitodin injection, were discovered during the 2023 RBPMS sampling. The products were said to have been manufactured by the same manufacturer, and all the products bear the same falsified NAFDAC Reg. No. A4-9566.
It is essential to highlight that these products identified in this alert are confirmed as falsified because they are not present in the NAFDAC-registered products database.
Oxytocin is a naturally occurring hormone and neuropeptide, also available as a pharmaceutical drug, primarily used to induce or strengthen labor, control postpartum bleeding, and support lactation. It binds to oxytocin receptors on the smooth muscle of the uterus, activating a G-protein-coupled receptor pathway. This leads to increased intracellular calcium levels, causing uterine contractions.
Risk Statement
Using unregistered or falsified Oxytocin injections poses serious health risks to both mothers and newborns. These products may contain incorrect doses, no active ingredient, or harmful contaminants, leading to ineffective uterine contractions, postpartum hemorrhage (PPH), and even maternal death. Poor-quality oxytocin may also delay or fail to stop bleeding after childbirth, increasing the need for emergency interventions like blood transfusions or surgery.
Product details
The details of the falsified products are as follows:
Product Name: Gold Vision Oxytocin injection 10IU (OXYTOCIN INJECTION), A-tocin injection, Extocin Injection, and Claxitodin injection.
Fake NAFDAC Reg. No: A4-9566
Product Manufacturer: Anhui Hongye Pharmaceutical Co., Ltd., Fengyang East Road, Bengbu, Anhui Province, China.
Marketed by: Gold Vision Medicals, No.4 Range Avenue, Independence Layout, Enugu, Nigeria.
All NAFDAC zonal directors and state coordinators have been instructed to conduct surveillance and retrieve any of the falsified products if found within their zones and states in Nigeria.
Importers, distributors, retailers, healthcare professionals, and consumers are hereby advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of unregistered /falsified Oxytocin Injection. All medicinal products & medical devices must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322, or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Product Images

Extocin Injection

Claxitodin injection
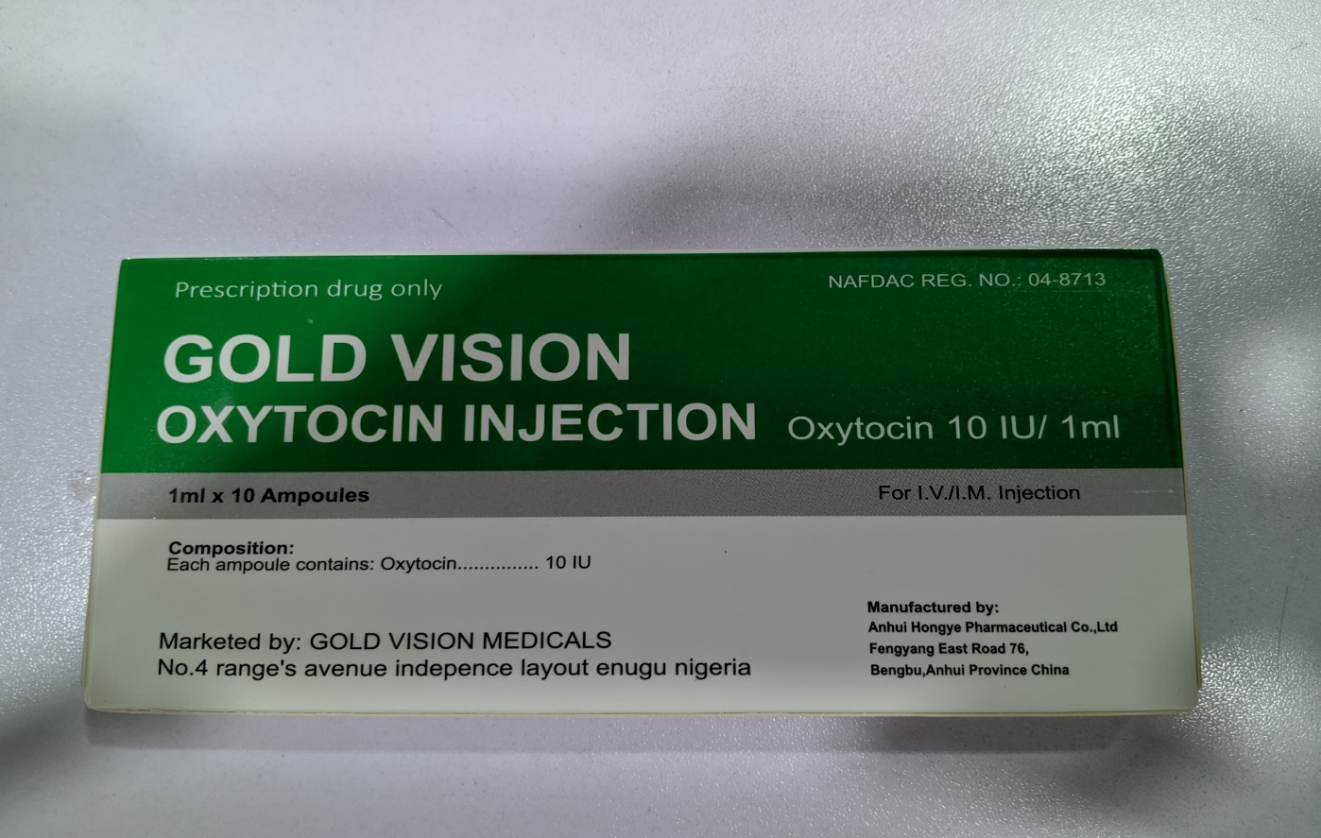
Gold Vision Oxytocin injection
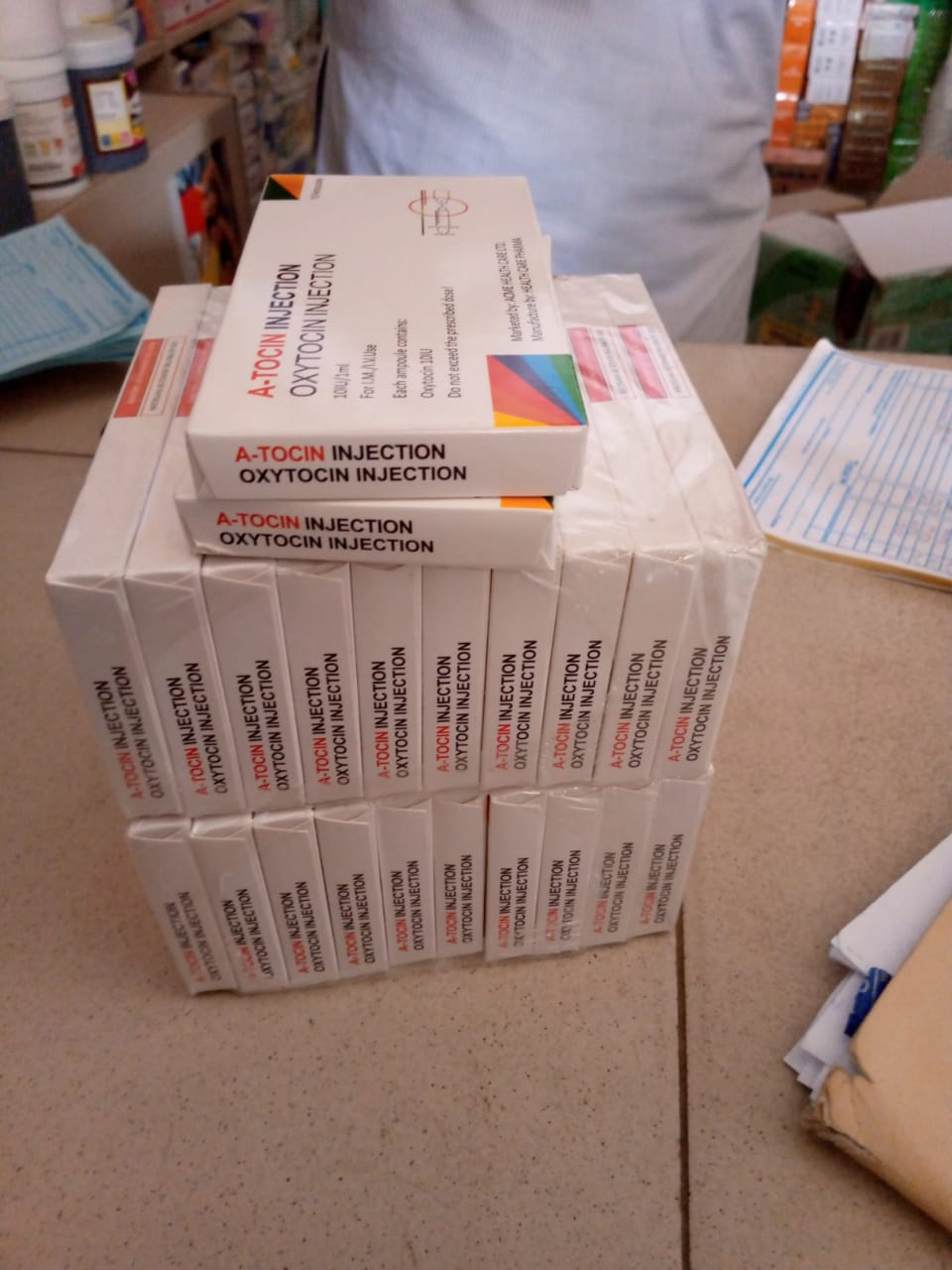
A-Tocin Injection
NAFDAC: Customer-focus, Agency-minded






