The National Agency for Food and Drugs Administration and Control (NAFDAC) is notifying the public about the sale of confirmed substandard ARTEMETRIN DS Tablets.
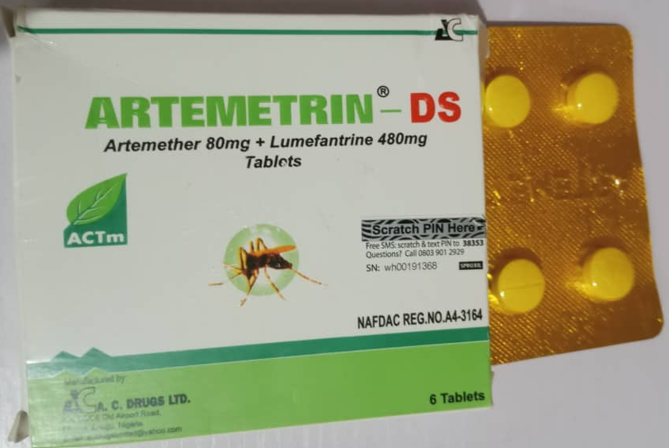
The ARTEMETRIN DS (Artemether/Lumefantrine) tablet (80mg/480mg) is registered product labelled as manufactured by A.C. DRUGS Ltd, Plot C5/C6 Old Airport Road, Emene, Enugu State, Nigeria. The product is sub-standard.
The product was initially subjected to thin-layer chromatography (TLC), which showed indications of irregularities in the results, thereby prompting further analysis at a WHO-prequalified laboratory. The results of the HPLC assay confirmed the following:
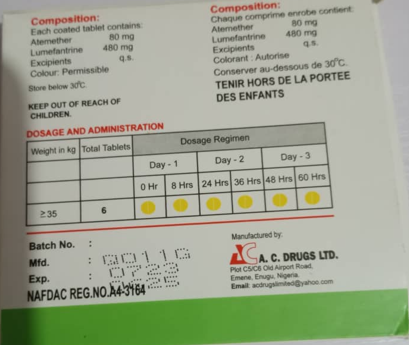
- ARTEMETRIN DS (Artemether/Lumefantrine) tablets (80mg/480mg) batch number Q011G with manufacturing date 07/23 and expiry date 06/25 contained only 59.2% Artemether and 71.2% Lumefantrine, which are outside the expected 90-110% limits. This is a violation of NAFDAC’s regulatory guidelines.
Genuine Artemether/Lumefantrine tablet is used to treat acute, uncomplicated malaria in both adults and children.
Risk
Poor-quality medicines can lead to treatment failures and antimicrobial resistance if the active pharmaceutical ingredient (API) is less than the specified dosage.
Also, the marketing of substandard, falsified, or unregistered medicines poses a great risk to public health.
Product Details
The details of the affected product are as follows.
Product Name | ARTEMETRIN DS (Artemether 80mg + Lumefantrine 480mg) |
Description | Tablets |
Stated manufacturer | A.C DRUGS Ltd, Plot C5/C6 Old Airport Road, Emene- Enugu State, Nigeria. |
Stated dose | (80mg/480mg) |
Stated NAFDAC Reg. No | A4-3164 |
Batch No. | Q011G |
Manufacture date | 07/23 |
Expiry date | 06/25 |
Packaging language | English/French |
Identified in | Nigeria |
Product Photo
Please see pictures of the substandard products below.
All NAFDAC zonal directors and state coordinators have been directed to carry out surveillance and mop up the substandard/falsified products within their zones and states.
NAFDAC implores distributors, suppliers, wholesalers, healthcare providers, and patients to exercise caution and vigilance within the supply chain to avoid distribution, sale, administration, and use of the products. All medical products must be obtained from genuinely authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
If you have any of the products mentioned above, please stop selling or using them immediately and submit your stock to the nearest NAFDAC office. If you or someone you know has used any of these products and experienced adverse reactions or events, we strongly recommend seeking immediate medical advice from a qualified healthcare professional.
Healthcare professionals and consumers are advised to report any suspicion of substandard and falsified medicines to the nearest NAFDAC office, NAFDAC on 0800-162-3322, or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of the medicinal product to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med-safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
NAFDAC………. Customer-focused, Agency-minded!!!
