Press Release by Director General, National Agency For Food And Drug Administration And Control, Prof Mojisola Christianah AdeyeyeNafdac Reaffirms Commitment To Enforce The Ban On Alcohol In Sachets And Small Plastic Bottles By December 2025
The National Agency for Food and Drug Administration and Control (NAFDAC) has reaffirmed its unwavering commitment to enforce the total ban on the production and sale of alcoholic beverages in sachets and small-volume PET/glass bottles (below 200ml) by December 2025, in line with the recent directive of the Senate of the Federal Republic of Nigeria. […]
WELCOME REMARKS BY THE DIRECTOR GENERAL (NAFDAC) AT THE WORLD BREASTFEEDING WEEK (1ST -7TH AUGUST, 2025) CAPACITY BUILDING EVENT FOR NAFDAC OFFICERS HELD ON 31ST JULY 2025 AT NAFDAC AUDITORIUM, ISOLO, LAGOS, DELIVERED BY DIRECTOR FSAN, MRS. EVA EDWARDS
Protocol It is with great pleasure that I welcome you all to the 2025 commemoration of World Breastfeeding Week, with the theme: “Prioritize Breastfeeding: Create Sustainable Support Systems.” This theme underscores the vital connection between breastfeeding and environmental sustainability. Breastfeeding is a natural, renewable, and environmentally friendly food source with minimal environmental impact — unlike […]
NAFDAC joins the Federal Ministry of Health and Social Welfare, nutrition partners, and the rest of the world in celebrating World Breastfeeding Week 2025

NAFDAC joins the Federal Ministry of Health and Social Welfare, nutrition partners, and the rest of the world in celebrating World Breastfeeding Week 2025 from 1st to 7th August. The theme for this year is “Prioritise Breastfeeding: Create sustainable support systems” Let us all join hands to protect, promote, and support breastfeeding by collaborating in […]
World Food Safety Day (WFSD) 2025

NAFDAC JOINS THE FEDERAL MINISTRY OF HEALTH AND SOCIAL WELFARE (FMOH&SW) TO COMMEMORATES THE WORLD FOOD SAFETY DAY 2025 The World Food Safety Day established by the United Nations (UN) General Assembly during its 73rd session and adopted on December 20, 2018, is celebrated annually on June 7. The Food and Agriculture Organization of the […]
Terms of use of NAFDAC Certificate of Product Registration
This is to inform the public that the National Agency for Food and Drug Administration and Control (NAFDAC) has developed a Terms of use of NAFDAC Certificate of Product Registration. This is to guide Market Authorization Holders (MAH) on the proper use of the NAFDAC Certificate of Product Registration and prevent abuse of same. MAHs […]
NAFDAC Refutes Claims of Approving Herbal Tea Promoting Smoking
The attention of the National Agency for Food and Drug Administration and Control (NAFDAC) has been drawn to a misleading video circulating on social media, falsely alleging that the Agency approved the registration of a herbal product claiming that “smoking is healthy” when used with their product. NAFDAC categorically refutes this claim as false, baseless, […]
Joint Press Briefing of NAFDAC and PCN on Coordinated Wholesale Centre in Kano- November 2024
PRESS BRIEF PROF MOJISOLA CHRISTIANAH ADEYEYE, DIRECTOR GENERAL, NATIONAL AGENCY FOR FOOD AND DRUG ADMINISTRATION AND CONTROL (NAFDAC) AND THE REGISTRAR, PHARM IBRAHIM BABASHEHU AHMED, PHARMACY COUNCIL OF NIGERIA ON RELOCATION OF OPEN DRUG MARKETERS TO COORDINATED WHOLESALE CENTRE (CWC) IN KANO: TO MAKE NIGERIANS HEALTHIER: CONTINUED FIGHT AGAINST SUBSTANDARD AND FALSIFIED MEDICINES (SFS) STATUS […]
Implementation of ICH Q1 and Q7 Guidelines

NAFDAC WORKSHOP ON ICH Q1 AND Q7 GUIDELINES AND ITS IMPLEMENTATION The National Agency for Food and Drug Administration and Control (NAFDAC), in partnership with Northeastern University, USA, and the International Council for Harmonization (ICH), successfully organized a workshop on ICH Q1 Stability Guidelines and ICH Q7 Good Manufacturing Practice (GMP) for Active Pharmaceutical Ingredients […]
Emergency use authorization of the Mpox vaccine by NAFDAC: A crucial step in Nigeria’s public health response
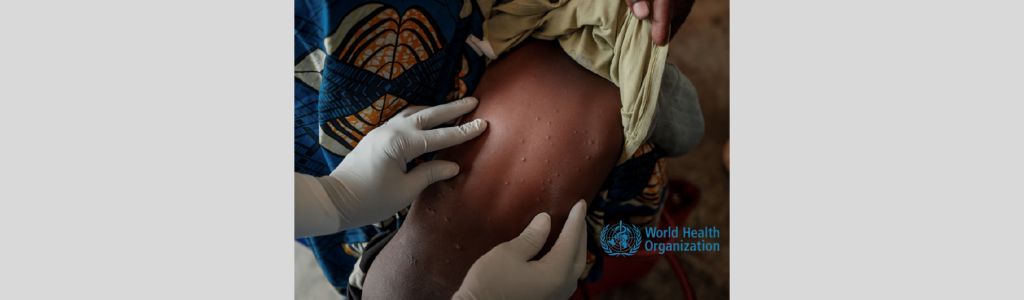
The 2022 Monkeypox (Mpox) outbreak, initially detected in the United Kingdom, posed a significant global health challenge. The World Health Organization’s declaration of a Public Health Emergency of International Concern underscored the urgency of the situation. In Nigeria, the National Agency for Food and Drug Administration and Control (NAFDAC) took proactive measures to ensure the […]
Unlocking the healthcare value chain: 2nd regional workshop on manufacturing Active Pharmaceutical Ingredients and excipients in Nigeria

Introduction Active Pharmaceutical Ingredients (APIs) are the essential components of pharmaceutical products responsible for the intended therapeutic effect.They form the backbone of pharmaceutical formulation and their quality directly impacts the safety and efficacy of the medicines which directly impacts public health. The National Agency for Food and Drug Administration and control (NAFDAC) has recognised the […]
