The National Agency for Food and Drugs Administration and Control (NAFDAC) is informing the public of the sale of counterfeit TANDAK injection 1.5g powder and water for injection, Manufactured by Intracin Pharmaceuticals PVT. LTD C-1, B-53, G.I.D.C Estate, Nadiad- 387001, Gujarat, India. This product was discovered in Gombe state, Nigeria, and reported to the Agency by Marcson Healthcare Ltd., the Marketing Authorisation Holder (MAH).
Tandak® injection of 1.5g powder is a co-formulation of Ceftriaxone 1000mg and Sulbactam 500mg. It is prescribed for use in the treatment of various types of bacterial infections. It fights against the microorganisms by preventing their growth and further spread of the infection. Ceftriaxone+Sulbactam 1000mg/500mg Injection should only be administered under the supervision of a healthcare professional.
Risk Statement
The illegal marketing of counterfeit medicines poses a risk to the health of people, since by not complying with the regulatory provisions, the safety, quality, and efficacy of the products are not guaranteed.
Product details
The differences between the original brand and the counterfeit brand are as follows:
S/N | ORIGINAL | COUNTERFEIT |
| BN: 23P24 | BN: 22P21 |
1 | Manufacturing: 08/2023 | Manufacturing: 05/2022 |
2 | Expiration date: 07/2025 | Expiration date: 08/2026 |
3 | Manuf. by Intracin Pharmaceuticals PVT. LTD | Manuf. by Intracin Pharmaceuticals PVT. LTD |
4 | Hologram on primary carton | Not included |
5 | A mobile authentication service label is present | It is photocopied/scanned |
6 | The cap on the vial is green | The Cap on the vial is white |
7 | The leaflet insert is present | The leaflet insert is missing |
8 | The logo of the exporter is Prime Pharmaceutical Pvt Ltd | The logo of the exporter is wrong and not consistent |
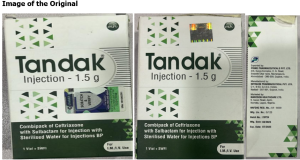
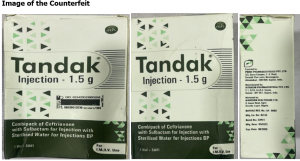
Product picture
NAFDAC has directed all zonal directors and state coordinators to carry out surveillance and mop up the counterfeit products within the zones and states.
Importers, distributors, retailers, healthcare professionals, and caregivers are hereby advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of the counterfeit products. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
NAFDAC………. Customer-focused, Agency-minded!!!
