The National Agency for Food and Drugs Administration and Control (NAFDAC) is notifying the public of a falsified batch of Giga-S Injection identified in Aba, Abia State.
The falsified batch was purchased from a renowned store located at Araria Market in Aba, Abia State.
Giga–S injection Ceftriaxone + Sulbactam Inj.1.5g IM/IV is indicated for the treatment of severe infections due to susceptible organisms including septicemia; pneumonia; and meningitis.
Product Details
The details of the original batch are as follows: –
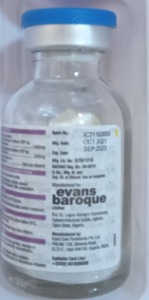
Product Name | Giga-S Injection |
Genuine Manufacturer | Inject Care Parenteral Pvt, Ltd. Plot No. 130, Silvassa Road, GIDC, Vapi- 396195 Gujarat, India. |
Batch number | 1C21163006 |
Expiry date | Sept. 2023 |
Date of manufacture | Oct. 2021 |
Product photo of Genuine (Expired Giga-S Injection)


The details of the Falsified are as follows:
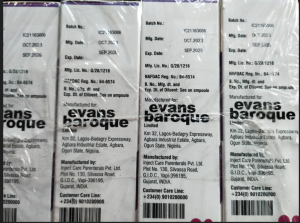
Product Name | Giga-S Injection |
Stated Manufacturer | Inject Care Parenterals Pvt, Ltd. Plot No. 130, Silvassa Road, GIDC, Vapi- 396195 Gujarat, India. |
Batch number | 1C21163006 |
Expiry date | Sept. 2026 |
Date of manufacture | Oct. 2023 |
Photo of the Falsified Giga-S Injection
Although this falsified batch product was identified in Abia state, it may likely have been distributed to other parts of the country through informal markets. Consequently, it is important to detect and remove it from circulation to prevent harm to patients.
NAFDAC, implores importers, distributors, retailers, and healthcare providers to always exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and administration or use of falsified or substandard medicinal products. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Members of the public in possession of the above product are advised to discontinue sale or use and submit stock to the nearest NAFDAC office.
If you have this counterfeit product, please DO NOT use it. If you, or anyone you know, have used this product, or suffered any adverse reaction/event after use, you are advised to seek immediate medical advice from a qualified healthcare professional.
Healthcare professionals and consumers are advised to report any suspicious substandard and falsified medicines to the nearest NAFDAC office, NAFDAC on
0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
NAFDAC……….Customer-focused, Agency-minded!!!
