The National Agency for Food and Drugs Administration and Control (NAFDAC) wishes to inform healthcare providers and the public of a report of a suspected counterfeit of Phesgo® 600mg/600mg, labeled with batch C3809C51.
The Marketing Authorization Holder (MAH) Roche received a complaint from a pharmacist reporting a suspected counterfeit Phesgo® 600mg/600mg, labeled with batch C3809C51. The product is reported to be brought in by a patient for administration.
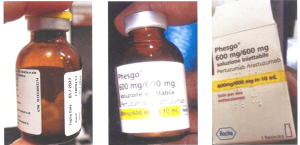
Although no sample was returned to Roche for investigation, only pictures displaying parts of a Phesgo® 600mg/600mg in a 10ml folding box and a labeled vial. The variable data of the folding box displayed in the picture shows EXXP: 01/2027, BN: C3809C51, MFD: 01/2024” and a serial number. There is a “Bollino” label on the folding box. Such labels are used in the Italian market. The vial is of brown glass and contains a white solid product. The vial seems to be unused. No pictures were available for the leaflet. Both the vial and folding box show information in Italian language.
The pictures of the suspected product were scrutinized and compared to genuine retained samples. The investigation identified the following significant differences between the complaint sample pictures and the genuine materials which confirmed the falsified status of the suspected counterfeit batch of Phesgo® 600mg/600mg;
- Batch number does not correspond to any genuine batch number of Phesgo®. This makes lot tracing impossible.
- Bollino sticker codes do not match the printed information.
- The tamper evidence label does not correspond to the genuine Roche tamper evidence label.
- Numerous incorrect placements of texts and logos on the folding box and the vial label, numerous obvious differences in fonts, printing quality, etc.
- The shape, size, dimensions and color of the glass vial are all incorrect.
- The stopper, flip-off cap, aluminum seal, and vial label all are different from the genuine materials.
- Chemical analysis is not possible because no physical sample is available for return. However, based on the photographs, the suspect product contains a solid/powder, whereas the genuine Phesgo® product contains a colorless liquid.
According to the MAH, there has been similar cases related to this issue. The photographs of the suspected counterfeit items show clear similarities with those of another case in Libya. In both instances, the counterfeits display the same batch number and variable data, serial number, and 2D matrix code, as well as identical Bollino labels.
Phesgo 600mg/600mg Solution for Injection is used to treat breast cancer. It works by killing the cancer cells and preventing their further growth.
Risk Statement
The illegal marketing of medicines or counterfeit medicines poses a risk to people’s health, as it does not guarantee the safety, quality, and efficacy of the products due to non-compliance with regulatory provisions.
Product details
The details of the counterfeit Phesgo® 600mg/600mg injection are as follows:
Product Name: Phesgo® 600mg/600mg injection
Stated Manufacturer: Roche
Batch Number: C3809C51
Manufacturing Date: 01/2024
Expiry Date: 01/2027
Product picture

All NAFDAC zonal directors and state coordinators have been directed to carry out surveillance and mop up the counterfeit products within the zones and states.
Importers, distributors, retailers, healthcare professionals, and caregivers are hereby advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale and use of counterfeit products. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, or call NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
NAFDAC………. Customer-focused, Agency-minded!!!
