The National Agency for Food and Drugs Administration and Control (NAFDAC) informs the healthcare providers and the public of a report of Confirmed Counterfeit of Herceptin in Nigeria.
The Marketing Authorization Holder (MAH) Roche received a complaint from a pharmacist reporting two units of suspected counterfeit Roche product, Herceptin 600 mg/5 ml.
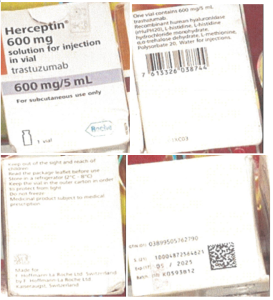
For the technical investigation, Roche only received pictures displaying parts of the Herceptin 600 mg/5 mL folding box, batch B3207B41. No pictures of vials and leaflets were available.
On investigation, the following were discovered for each product.
Herceptin 600 mg/5 mL, batch B3207B41
The Kaiseraugst/Switzerland Quality Control organization investigated the complaint sample pictures. The batch number does not exist for Herceptin, but the imprinted GTIN corresponds to the Roche common makeup MAK-21. Therefore, the displayed complaint sample was compared to a corresponding retain sample in the MAK-21 make-up presentation.
Several differences were detected, including non-existent batch number and wrong shelf life, different printing quality (e.g. colors, Roche log), and a different printing quality and font of variable data.
Batch Trace: The lot tracing was impossible because the lot number imprinted is not a genuine Roche batch number for Herceptin.
Chemical Analysis: Chemical analysis was not possible, as the physical complaint sample was not available for return. Nevertheless, the investigation of the provided pictures revealed clear evidence of counterfeit packaging material.
HERCEPTIN (trastuzumab) is a targeted cancer drug. It can be used to treat HER2-positive breast cancer that is either early-stage or advanced-stage/metastatic.
Risk Statement
The illegal promotion or sale of counterfeit medicines puts people’s health at risk as it does not ensure the safety, quality, and effectiveness of the products.
Product details
The details of the counterfeit products are as follows:
Product Name | Herceptin 600 mg/5 mL |
Stated Manufacturer | Roche
|
Batch Number | B3207B41 |
Manufacturing Date | 03/2023 |
Expiry Date | 03/2025 |
Product picture
All NAFDAC zonal directors and state coordinators have been directed to carry out surveillance and mop up the counterfeit product within the zones and states.
Importers, distributors, retailers, healthcare professionals, and caregivers are hereby advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale and use of counterfeit product. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, or call NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
NAFDAC………. Customer-focused, Agency-minded!!!
