The National Agency for Food and Drug Administration and Control (NAFDAC) hereby notifies the public of falsified Type 1 and 2 batches of POSTINOR 2 (Levonorgestrel 0.75mg) product in circulation. A report was received from the Society of Family Health (SFH), the MAH, which confirmed that their company did not import that product batch.
The noticeable difference was found to be as follows:
The font size of the text on the pin verification sticker appears smaller and has a wrong spelling of the word Veify instead of Verify on the fake; meanwhile, the text font on the sticker of the original appears bigger and more visible. There is also a wrong spelling behind the pack of the fake Distnibuted in Nigeria instead of distributed in Nigeria
Postinor-2 (Levonorgestrel 0.75mg) is a brand of emergency contraceptive pill (ECP) containing the active ingredient levonorgestrel.
Product Details
The falsified Product details are as follows:
ORIGINAL PRODUCT | COUNTERFEIT PRODUCT (Type 1) | COUNTERFEIT PRODUCT (Type 2) |
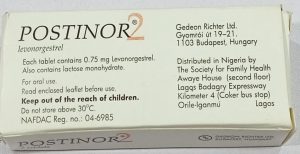
Product Name: Postinor 2 Batch No: T32458H Mfg. Date: 02/2023 Exp. Date: 02/2027 NRN NO: 04-6985
| Product Name: Postinor 2 Batch No: T36184B Mfg. Date: 08/2024 Exp. Date: 08/2028 NRN NO: 04-6985
| Product Name: Postinor 2 Batch No: 332 Mfg. Date: 03/2023 Exp. Date: 02/2027 NRN NO: 04-6985
|
Risk Statement:
Due to the potential presence of incorrect, substandard, or harmful ingredients, improper dosages of levonorgestrel, and a lack of sterile manufacturing conditions, poses significant risks to individual health and public safety. The risks of administering falsified Postinor 2 (Levonorgestrel 0.75mg) include failure of contraceptive effect, toxic or harmful contaminants, unpredictable side effects, delayed or missed opportunity for genuine emergency contraception, and potential long-term reproductive health impact. unexpected side effects: Unknown substances can trigger allergic reactions, organ damage, or death. Counterfeit medicines are unregulated, untested, and illegal, making their safety and efficacy impossible to guarantee. Patients should only obtain Postinor-2 from verified pharmacies or licensed healthcare providers.
Although Investigations are still ongoing regarding the source of the falsified product, All NAFDAC zonal directors and state coordinators have been directed to carry out surveillance and mop up the falsified product of type 1 and 2 postinor 2 (Levonorgestrel 0.75mg) within the zones and states.
Distributors, retailers, healthcare professionals, and caregivers are advised to exercise caution and vigilance in the supply chain to prevent the distribution, sale, and use of falsified products. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
Product Image:
Original Product


Counterfeit Product (Type1)


Counterfeit Product (Type2)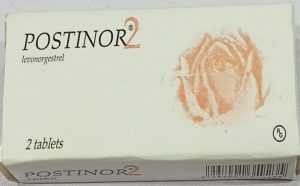

NAFDAC………. Customer-focused, Agency-minded!!!
