The National Agency for Food and Drugs Administration and Control (NAFDAC) informs the healthcare providers and the public of a report of Confirmed Counterfeit of Mabthera 500mg/50ml with batch number N7458B07 in Nigeria.
The Marketing Authorization Holder (MAH) Roche received a call from a patient to enquire the genuineness of the product before purchasing it. The product pack was observed to have some discrepancies from the Mabthera distributed by Roche in Nigeria.
Roche has further investigated the complaint sample picture. The displayed complaint samples were compared to a batch corresponding with a retain sample in Turkish make-up presentation. It was confirmed that the genuine product of Mabthera vials 500mg/50ml, batch number N7458B07 was distributed by Roche to Turkiye in February 2021 which expired in November 2022 and was never shipped into Nigeria.
Significant differences were found regarding the displayed packaging material. The folding boxes and the Tamper Evident labels do not correspond to genuine Roche MabThera packaging material. The English text printed on the packaging material seems to be an automatic translation from Turkish to English (e.g. with the use of a translation tool).
Chemical analysis was not possible, as the physical complaint sample was not provided for investigation, Roche only received pictures displaying parts of the Mabthera 500mg/50ml pack, with batch number N7458B07. No pictures of vials and leaflets were available.
MabThera can be used for the treatment of children and adolescents, 6 months of age and older, with non-Hodgkin’s lymphoma, specifically CD20 positive diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma (BL)/Burkitt leukaemia (mature B-cell acute leukaemia) (BAL) or Burkitt-like lymphoma (BLL).
Risk Statement
The illegal promotion or sale of counterfeit medicines puts people’s health at risk as it does not ensure the safety, quality, and effectiveness of the products.
Product details
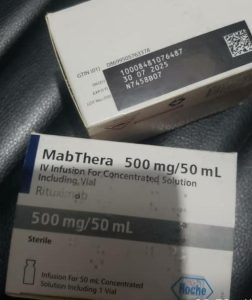
The details of the counterfeit products are as follows:
Product Name | Mabthera 500mg/50ml |
Stated Manufacturer | Side not provided in photo |
Batch Number | N7458B07 |
Manufacturing Date | Not provided on pack |
Expiry Date | 07/2025 |
The correct manufacturing site for genuine Mabthera vial 500mg/50ml is Roche Diagnostics GmbH, Galencial Plant Germany, Sandhofer Strasse 116 68305, Mannheim, Germany.
Product picture
All NAFDAC zonal directors and state coordinators have been directed to carry out surveillance and mop up the counterfeit product within the zones and states.
Importers, distributors, retailers, healthcare professionals, and caregivers are hereby advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale and use of counterfeit product. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, or call NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
NAFDAC………. Customer-focused, Agency-minded!!!
