The National Agency for Food and Drug Administration and Control (NAFDAC) is alerting the public of one batch of falsified OXYCONTIN 80mg (oxycodone hydrochloride) detected in the unregulated market in Switzerland. This was reported to the World Health Organization (WHO) by the genuine manufacturer, MUNDIPHARMA, in February 2025. The falsified product imitates the genuine OXYCONTIN 80mg authorized for sale in Poland.
Genuine OXYCONTIN (oxycodone hydrochloride) is a semi-synthetic opioid indicated for the treatment of moderate to severe pain.
Laboratory tests of samples for the falsified product were conducted by the Drug Information Centre in Zurich, Switzerland, according to WHO. DIZ’s drug-checking service determined that the tablets did not contain oxycodone, but a synthetic opioid likely to be a nitazene compound.
Nitazene derivatives (e.g., metonitazene, isotonitazene, fluonitazene) are potent synthetic opioids, primarily used in research due to their high addiction potential and severe side effects. These substances can be hundreds of times stronger than oxycodone, posing a high overdose risk. Limited information is available on their risks, toxicity, side effects, and long-term consequences.
The identified product in this alert is confirmed as falsified on the basis that it deliberately/fraudulently misrepresented its identity, composition, or source. The falsified product imitates OXYCONTIN 80mg manufactured and marketed by MUNDIPHARMA in the Polish market. MUNDIPHARMA has confirmed that the product was falsified and was not produced by their company.
Risk
This falsified product has been found to contain undeclared nitazene compounds, which pose a significant risk due to the high likelihood of adverse events, even in small doses. Nitazenes produce similar effects to other opioids. Their high potency carries a high risk of overdose and death. Using nitazene derivatives has been linked to several deaths. Mixing them with other depressants like alcohol or benzodiazepines can be very dangerous, leading to severe effects like respiratory depression, low blood pressure, coma, or even death.
This falsified product poses a particular risk to individuals with substance use disorders who may perceive this falsified product as a safe and quality-assured medicine.
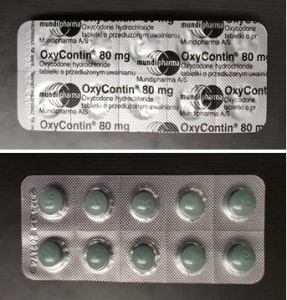
The following visible discrepancies were observed on the falsified product
- The placement of the batch and expiry dates on the counterfeit product is incorrect.
- On the falsified product the batch and expiry date are visible on the front side of the blister strip.
- Genuine OXYCONTIN has the batch and expiry date visible on the back of the blister strip.
- On the falsified product the expiry date is on the left and the batch number is on the right.
- Genuine OXYCONTIN has the batch number on the left and the expiry date on the right.
Product details
Details of the falsified product are as follows:
Product Name | OxyContin 80mg |
Stated manufacturer | Mundipharma A/S |
Batch | 262174 |
Expiry date | 12/2025 |
Identified in | Switzerland |
Available photos
|  |
All NAFDAC zonal directors and state coordinators have been instructed to conduct surveillance and retrieve any falsified products found within their zones and states in Nigeria.
Importers, distributors, retailers, healthcare professionals, and consumers are hereby advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of falsified OXYCONTIN tablets. All medical products/ medical devices must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
NAFDAC………. Customer-focused, Agency-minded!!!
