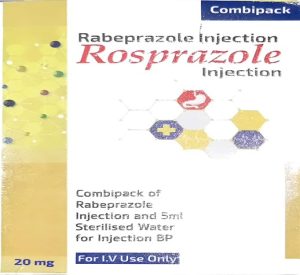
The National Agency for Food and Drug Administration and Control (NAFDAC) is alerting the public about a falsified Rosprazole (Rabeprazole Injection) 20mg with a fake NAFDAC Reg. No. C4-0150.
Genuine Rabeprazole Injection (20mg) is a proton pump inhibitor (PPI) used in hospital settings to manage various acid-related gastrointestinal conditions especially Gastroesophageal reflux disease (GERD), to reduce stomach acid and heal esophageal damage, Peptic ulcers Including those with bleeding or severe erosions and Zollinger-Ellison syndrome, a condition causing excessive stomach acid production.
The identified product in this alert is confirmed as falsified on the basis that it deliberately and fraudulently misrepresented its identity. Investigation revealed that the stated NAFDAC Registration Number on the falsified product belongs to another product: Amlodipine tablet (Amlodipine Besilate).
Risk
Using falsified or counterfeit Rosprazole (Rabeprazole e injection)20mg poses significant health risks, primarily due to the absence of Rabeprazole (or an inadequate amount), which may be poorly manufactured and contain harmful chemicals or contaminants, which could result in organ failure, allergic reactions, or even death.
Product details
Details of the falsified product are as follows:
Product Name: Rosprazole Injection (Rabeprazole Injection 20mg)
Batch No: PLED23108
Mfg. Date: 12/2023
Exp. Date: 11/2026
NRN NO: C4-0150
Manufacturer: Nil
Manufactured for: Celeste Farmaceutical Inc., 2063, Rabari Vas Khoraj, Gujarat, India
Imported by: Rostrec Pharmacy and Stores No. 98 Oron Road, Uyo, Akwa Ibom State
All NAFDAC zonal directors and state coordinators have been instructed to conduct surveillance and retrieve any falsified products found within their zones and states in Nigeria. Importers, distributors, retailers, healthcare professionals, and consumers are hereby advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of falsified Rosprazole (Rabeprazole Injection) 20mg. All medical products & medical devices must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322, or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng


NAFDAC………. Customer-focused, Agency-minded!!!
