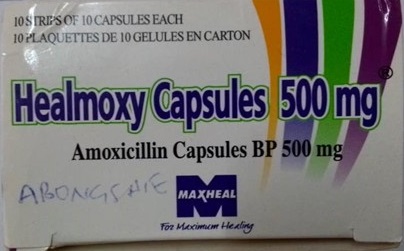
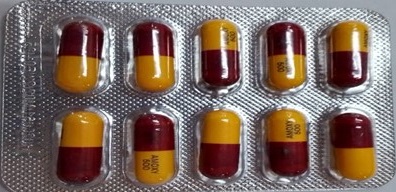
The National Agency for Food and Drug Administration and Control (NAFDAC) hereby notifies the public of the falsified batches of Healmoxy Capsules 500mg, manufactured by Maxheal Pharmaceuticals (India), with batch numbers 023011 and H02605, found in Cameroon and H02605 in the Central African Republic (CAR). The brand is registered and imported by Nkoyo Chemist, located at 23 Agbonyi Street, Aguda, Surulere, Lagos.
Laboratory analysis of the samples by the WHO revealed that the product lacks an active pharmaceutical ingredient (API) and has inconsistent formats for the manufacturing and expiry dates.
The active ingredient in Healmoxy 500mg Capsules is Amoxicillin, specifically in the form of Amoxicillin Trihydrate. Amoxicillin capsule 500mg is a broad-spectrum antibiotic belonging to the penicillin class. It is a prescription medicine primarily used against various bacterial infections, including those affecting the respiratory tract, urinary tract, skin, and gastrointestinal system.
Product Details
The falsified product details are as follows.
S/N | Manufacturing Date | Expiry Date | Batch |
1 | 01/23 | 01/26 | HO26051 |
2 | 11/01/2024 | 10/01/2027 | 023011 |
3 | 03/24 | 02/27 | H02605 |
4 | 02/2022 | 01/2025 | H026051 |
5 | 17/06/22 | 18/07/25 | 023011 |
Risk Statement:
The administration of Falsified drugs poses significant risks to individual health and public safety. As earlier mentioned, the product lacks an active pharmaceutical ingredient (API). This can result in the drug lacking its therapeutic agent, rendering it useless for treatment, its use can lead to ineffectiveness or toxicity, potentially causing overdose or resistance, and harmful substances containing toxic or contaminated ingredients, which can lead to unexpected side effects. Unknown substances can trigger allergic reactions, organ damage, or death.
Although an investigation by PMS Lagos into the import documents of Nkoyo Chemist Ltd reveals that the batches were never imported into Nigeria, NAFDAC urges importers, distributors, retailers, healthcare providers, and patients to exercise caution and vigilance throughout the supply chain. This is essential to prevent the importation, distribution, sale, administration, or use of falsified products. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of substandard and falsified medicines to the nearest NAFDAC office, on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of the affected product to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng


NAFDAC………. Customer-focused, Agency-minded!!!
