The National Agency for Food and Drugs Administration and Control (NAFDAC) is informing the public of the presence an unregistered batch of Tarivid 200mg in Nigeria.
The product was discovered by the Agency’s Post Marketing Surveillance Directorate Officers in Lagos State during a routine surveillance activity conducted in Onipan area of Shomolu Local Government Area, Lagos State.
On investigation, it was confirmed by a Representative of the product’s Marketing Authorization Holder (SANOFI) that the unregistered batch identified during the surveillance was not intended for the Nigerian market, but rather for Pakistan market. This conclusion is as a result of a review conducted on the batch by Sanofi’s Anti-Falsified and Illicit Trafficking (AFIT) Central Lab. It was also stated that the batch is not authorized for distribution in the Nigerian and does not fall under the scope of validly registered product with NAFDAC.
Tarivid is a brand of Ofloxacin. Ofloxacin is an antibacterial agent used for the treatment of bacterial infections in many parts of the body, including the respiratory tract, kidney, skin, soft tissue, and urinary tract.
Risk Statement
The sale of unregistered medicines poses a risk to people’s health, since by not complying with the regulatory provisions, the safety, quality, and efficacy of such products are not guaranteed.
Product Details
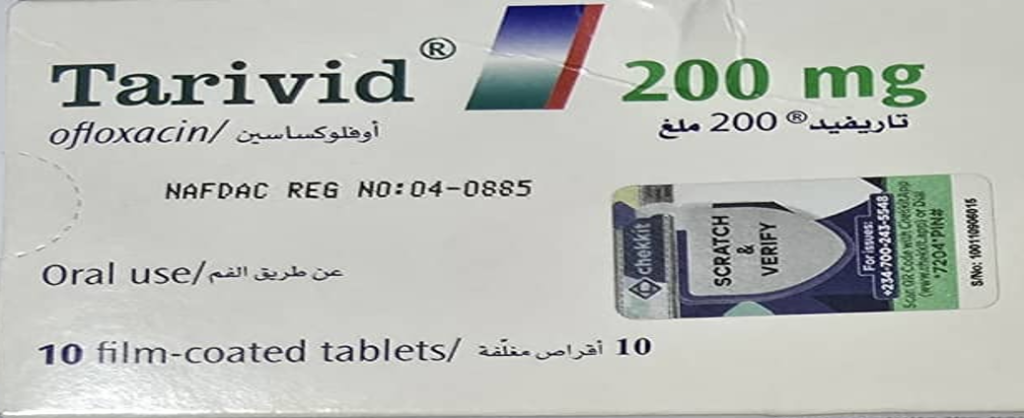
The details of the unregistered product are as follows;
Product Name | Tarivid (Ofloxacin 200mg) |
Product Manufacturer | Sanofi |
Manufactured Date | Sep. 2023 |
Expiry Date | Aug. 2028 |
Stated NRN | AL016 |
Product Photos

All NAFDAC zonal directors and state coordinators have been directed to carry out surveillance and mop up the counterfeited product within the zones and states.
Distributors, retailers, healthcare professionals and caregivers are hereby advised to exercise caution and vigilance within the supply chain to avoid the distribution, sale and use of the unregistered product. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
NAFDAC………. Customer-focused, Agency-minded!!!
