The National Agency for Food and Drug Administration and Control (NAFDAC) is alerting the public about the confirmed circulation of counterfeit Herceptin® 600mg/5ml (Trastuzumab Solution for Injection) in Ghana. The counterfeit product, identified by batch number A8519, does not correspond to any authentic batch produced and marketed by Roche.
This counterfeit was reportedly presented by a patient at a hospital in Kumasi, who claimed to have purchased it in Nigeria.
Herceptin 600mg/5ml injection is used to treat breast cancer in cases where the tumor tests positive for HER2. It may be administered alone or alongside other treatments for breast cancer, such as an aromatase inhibitor for hormone receptor-positive breast cancer or a taxane (e.g., paclitaxel or docetaxel).
How to Identify Counterfeit Herceptin® 600mg/5ml
The following features can recognize the counterfeit Herceptin® 600mg/5ml solution for injection:
- Batch Number: A8519 is not a valid Roche batch and is not traceable in Roche’s manufacturing and distribution systems.
- Packaging Differences:
- Font type inconsistencies.
- Misplacement of label text and variable data.
- Tamper-evident seals do not match those used on authentic Roche packaging.
Possible Risk
- The counterfeit product may contain substandard, falsified, or no active pharmaceutical ingredient (trastuzumab), rendering treatment ineffective.
- Unknown chemical contents pose a risk of adverse drug reactions, including allergic reactions, systemic toxicity, or other severe health outcomes.
- Use of falsified oncology products compromises the integrity of therapeutic regimens, potentially leading to disease progression or mortality.
Product Details
The details of the counterfeit Herceptin are as follows.
Product Name | HERCEPTIN 600mg/5ml injection |
Stated Manufacturer | Roche Products Limited |
Batch number | A8519 |
Expiry date | 12/2026 |
Date of manufacture | 01/2024 |
Product Photo
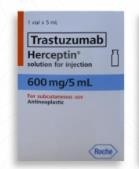
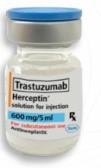
Fig 1: Genuine Herceptin Solution for Injection
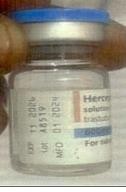
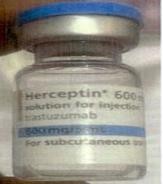
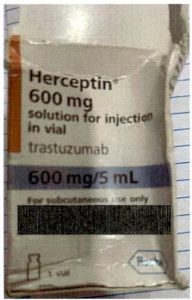


Fig. 2: Counterfeit Herceptin Solution for Injection
All NAFDAC zonal directors and state coordinators have been directed to carry out surveillance and mop up the counterfeit Herceptin® 600mg/5ml solution for injection if found within the zones and states.
Distributors, retailers, healthcare professionals, and caregivers are hereby advised to exercise caution and vigilance within the supply chain to avoid the distribution, sale, and use of the counterfeit product. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
Healthcare professionals and consumers are advised to report any suspicion of sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322, or via email: sf.alert@nafdac.gov.ng
Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office, or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng
Furthermore, note that this notice will be uploaded to the WHO Global Surveillance and Monitoring System (GSMS).
NAFDAC………. Customer-focused, Agency-minded!!!
