Home » Pharmaceutical Traceability
Pharmaceutical Traceability
NAFDAC was established by Decree No. 15 of 1993 as amended by the ACT Cap N.1 LFN 2004 to regulate and control the importation, exportation, manufacture, advertisement, distribution, sale and use of food, drugs, cosmetics, medical devices, bottled water and chemicals. The ACT places the burden of drug distribution, sale, use and security on the Agency. The goals of NAFDAC regulatory control include, but are not limited to the following:
- Safeguard the health of the population.
- Establish a prevention, detection and response mechanism and minimize substandard falsified medicines, narcotics, foods and other regulated products.
- Improve supply chain security and reverse logistics.
- Establish a traceability system to accomplish the above goals.
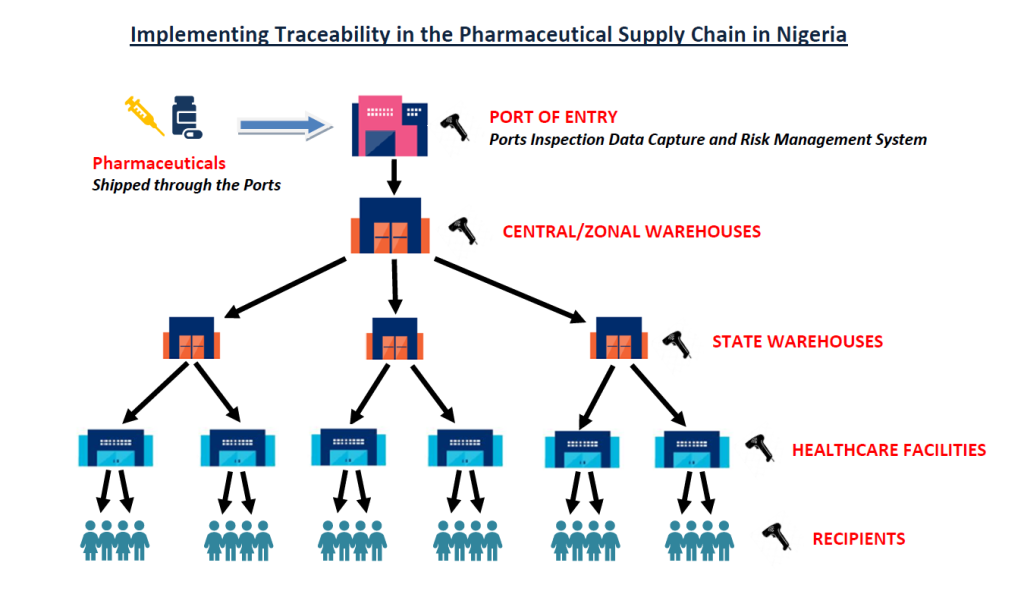
NAFDAC aims to have full visibility over all products moving within the pharmaceutical supply chain in Nigeria and will publish and enforce Regulations and guidelines to achieve this objective. NAFDAC expects traceability to be operational in the Nigerian Pharmaceutical Supply Chain by the end of the year 2024.
NAFDAC’s traceability implementation strategy is in line with the Nigeria National Traceability Strategy Policy document published by the Federal Ministry of Health in May 2020 and the Call to Action for the “Africa Strategy for Pharmaceutical Traceability” signed by 25 African Regulatory agencies and six (6) health financing and donor organizations.
Traceability will:
- Provide visibility of product from plant to patient;
- Promote trust in the pharmaceutical sector and healthcare system;
- Provide increased opportunity for trade of domestically manufactured pharmaceuticals;
- Increase quality of data to support pharmacovigilance;
- Decrease the presence of substandard and falsified (SF) medicines;
- Enable efficiencies across the supply chain; and ultimately;
- Increase patient safety.
NAFDAC has developed a 5-Year Traceability Implementation plan in line with these objectives to achieve supply chain visibility and strengthen its pharmacovigilance activities against the scourge of Substandard and Falsified Medicines and Medical Devices by the end of the year 2024.
We invite you all to take this laudable journey with us.
Signed.
See posters:


