OVERVIEW OF THE DIRECTORATE
A. Introduction
The Central Drug Control Laboratory (CDCL) of the National Agency for Food and Drug Administration and Control (NAFDAC) is one of the seven official reference laboratories established to undertake quality control testing of medicines submitted to Nigeria’s National Medicines Regulatory Authority, the National Agency for Food and Drug Administration and Control (NAFDAC) for regulatory purposes such as Licensing, market surveillance, enforcement, investigation etc. and other purposes.
The Central Drug Control Laboratory is located at Number 8/10 Merret Road, Medical Compound, Yaba, Lagos, Nigeria.
The Central Drug Control Laboratory, Yaba is responsible for regulatory testing of a wide range of products such as medicinal products, veterinary medicines, herbal preparations, cosmetics etc.
The Central Drug Control Laboratory commenced operation in 1978 under the department of Food and Drug Administration of Nigeria’s Federal Ministry of Health. It later became the Central Drug Quality Control and Vaccine Laboratory when NAFDAC was established in 1993 and in 2009 as Central Drug Quality Control Laboratory (CDQCL) with the vaccine unit established as a separate and independent laboratory. In 2013, it assumed its current status as Central Drug Control Laboratory (CDCL).
In 2018, following an organizational restructuring for greater efficiency, Laboratory Services Directorate Drug and Vaccines was created, which comprised three of the seven (7) laboratories in NAFDAC – CDCL and two others (NCLVB and MAL). In 2022, the National Control Laboratory for Vaccines and other Biologicals (NCLVB) became an independent laboratory and Directorate known as the Vaccines, Biologics and Medical Devices, Laboratory Services Directorate (VBM-LSD)., and therefore CDCL alongside MAL became Laboratory Services – Drug Directorate.
B. Certifications, Accreditation
CDCL provides reliable and accurate analytical laboratory services in line with WHO Good Practices for Pharmaceutical Quality Control Laboratories (GPPQCL), WHO Global Benchmarking Tool (GBT) requirements and ISO/IEC 17025:2017 Standards.
In demonstration of the laboratory’s commitment to establishing and implementing Quality Management Systems (QMS) in line with global standards, the Laboratory recently achieved the WHO Pre-Qualification Status.
CDCL demonstrates its technical competence in seventeen (17) testing scopes as it has sustained the ISO 17025:2017 Accreditation consistently for almost a decade. NAFDAC, of which the Laboratory is a part, operates its management system in line with Quality Management System standards evidenced by its ISO 9001:2015 Certification. Furthermore, the Agency within which CDCL operates, attained the WHO Global Benchmarking Tool (GBT) Maturity Level 3, which is the status of a stable, well-functioning regulatory system.
C. Scope and Areas of Expertise
CDCL specializes in quality control testing for Finished Pharmaceutical Products and Active Pharmaceutical Ingredients (API). It provides these services through competent, multi-disciplinary workforce using established validated or standard methods in carrying out its tests. These processes are in accordance with requirements of the WHO Good Practices for Pharmaceutical Quality Control Laboratories (GPPQCL), WHO Global Benchmarking Tool (GBT) and ISO/IEC 17025:2017 Standards.
Type of Analysis | Finished Products | Active Pharmaceutical Ingredients |
Physical/chemical analysis | pH, uniformity of dosage unit (mass, content), Mass uniformity (uniformity of weight), disintegration, loss on drying, water content (Karl Fischer), dimensions, , viscosity, specific gravity, optical rotation, Net Content (Fill Volume/Deliverable volume), Residue on ignition, Friability, Hardness, Physical description | melting point, pH, loss on drying, water content (Karl Fischer), viscosity, ash content, specific gravity, optical rotation, Physical description. |
Identification | HPLC, Spectrophotometric, IR, TLC, and basic tests | HPLC, spectrophotometric, IR, TLC, and basic tests |
Dissolution | Dissolution | NA |
Assay, impurities and related substances | HPLC (UV-VIS detection, DAD, RID, fluorescence), spectrophotometry, GC, polarimetry, volumetric titrations. determination of related substances/impurities and degradation products | HPLC (UV-VIS detection, DAD, RID, fluorescence), spectrophotometry, GC, polarimetry, volumetric titrations. determination of related substances/impurities and degradation products |
Microbiological tests | sterility test, microbial limit tests and microbial assay. | microbial limit tests, microbial assay |
Bacterial Endotoxin Testing | Bacterial endotoxin test (LAL and gel clot method), test for pyrogen. | NA |
Biological Activity (Organ Bath) | Biological Activity | NA |
Ocular Activity | Ocular activity (Eye drops) | NA |
Dermal toxicity | Dermal toxicity | NA |
D. Equipment and Facilities
To undertake quality control activities for regulatory requirements and other purposes, CDCL uses state of art equipment and facilities for testing purposes and to ensure integrity of the samples submitted for quality control testing are maintained.
The Laboratory carries out sample management and generates test reports with the aid of a validated Laboratory Information Management System (LIMS). Environmental conditions for testing and sample storage are appropriately controlled and monitored by electronic data loggers.
Wide range of updated and modern equipment such as the High Performance Liquid Chromatography, Fourier Transform Infra Red, Dissolution Testers, Gas Chromatography, Karl Fischer Titrator, Melting Point Apparatus etc See full list of equipment list.
CDCL; a Regional Centre for Regulatory Excellence (RCORE) in Quality Assurance
In 2014, CDCL was designated a Regional Center for Regulatory Excellence in Quality Assurance and Quality Control of Medicines. It has since built capacity and expertise in this regard. As a result, many organizations have benefited from training programs offered by the Laboratory in Quality Management Systems and other key technical processes delivered in both classroom and hands-on style by the Laboratory. These organizations include National Regulatory Agencies, Government military and research organizations, Higher educational institutions etc. Further details on the RCORE Program, curriculum, and program structure.
Pictures

Front review on the Central Drug Control Laboratory

Side-view of the CDCL Laboratory Complex

High Performance Liquid Chromatography (HPLC) II Laboratory

Pharmaceutical Control (PCON) Laboratory
Summary of Laboratory Analytical Performance 2021, 2022, 2023 & 2024
| YEAR 2021 | YEAR 2022 | YEAR 2023 | YEAR 2024 |
Total Number of Samples received for the year under review | 9,396 | 10,854 | 8301 | 9091 |
Number of Samples Analyzed | 8,803 (93.69%) | 10,394 (95.74%) | 8165 (98.36%) | 7962 (87.78%) |
Number of samples pending for the year under review | 593 (6.31%) | 460 (4.26%) | 136 (1.64%) | 1129 (12.22%) |
Table 1: Laboratory Analytical Performance from January to December for 2021, 2022 2023 and 2024
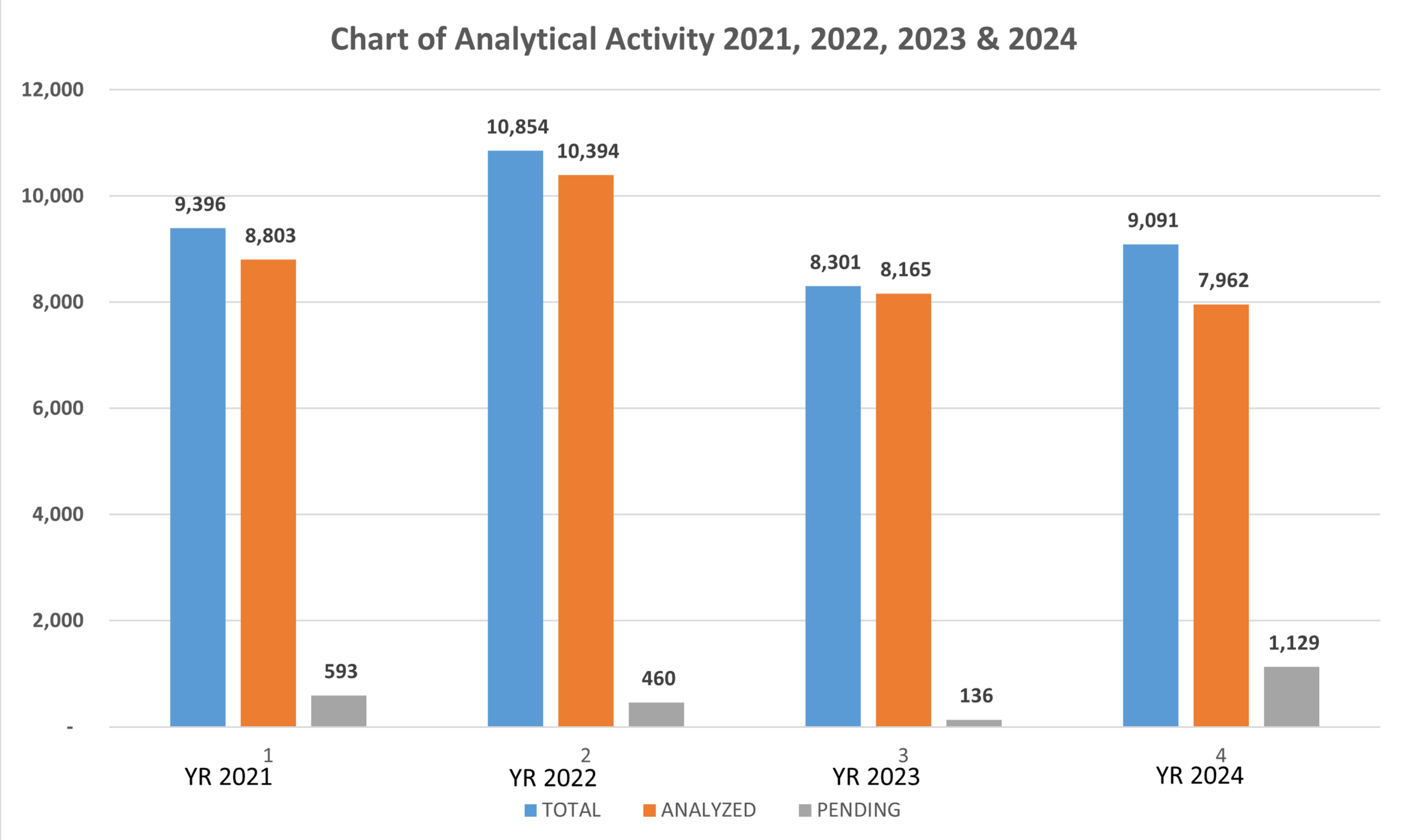
Graph 1: Graphical presentation of the Laboratory Analytical Performance from January to December for 2021, 2022, 2023 and 2024
Laboratory Staff Strength with Distribution of Available Expertise
SN | Discipline | Number |
1. | Chemists | 18 |
2. | Pharmacists | 9 |
3. | Microbiologists | 10 |
4. | Biochemists | 9 |
5. | Doctors (Veterinary Medicine) | 2 |
6. | Laboratory Assistant | 6 |
7. | Engineer | 7 |
8. | Statistician | 1 |
9. | Science Lab technologists | 3 |
10. | Pharmaceutical Scientist | 1 |
11. | Pharmacologist | 1 |
12. | Botanist | 1 |
13. | Others (Administrative officer, Accountants, Secretaries etc) | 7 |
| Total | 75 |
Table 2: Tabular presentation of Laboratory Personnel with respective expertise available.
