NAFDAC ELECTRONIC CLINICAL TRIAL APPLICATION PLATFORM (NAFDAC-eCTAP)
NAFDAC has launched the NAFDAC Electronic Clinical Trial Application Platform (NAFDAC-eCTAP) in partnership with NUVOTEQ and support from the Gates Foundation. This online platform is designed to provide a seamless and transparent system for submitting clinical trial applications, amendments, and progress/final reports.
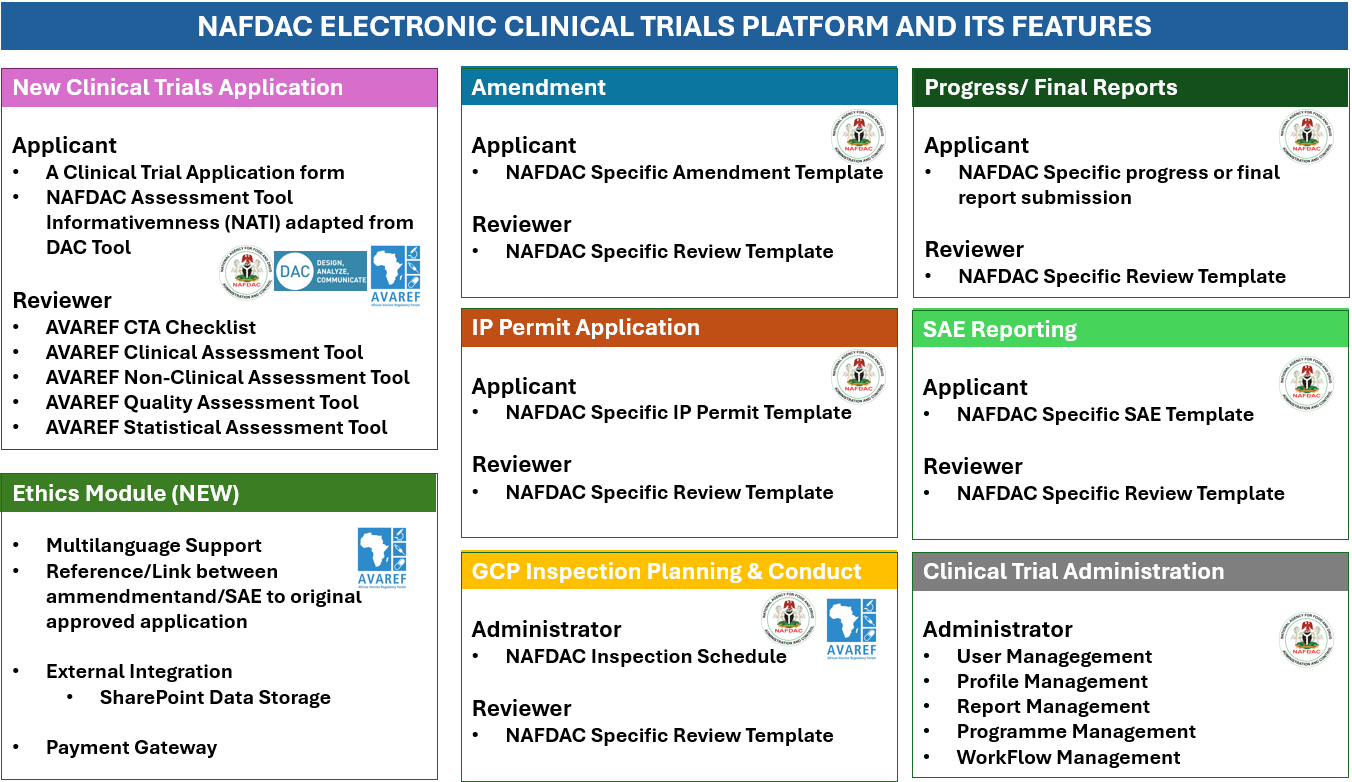
It also facilitates the importation of investigational medicinal products (IMP) for clinical trials. The NAFDAC-eCTAP aims to reduce turnaround times, mitigate challenges of paper-based submissions, and improve applicant’s ability to track progress. It is the first electronic application platform of its kind in Africa and can be accessed on the NAFDAC website or through the link below:
